-
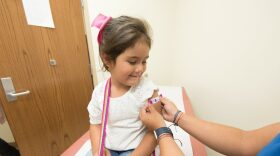
Young children would still be required to get vaccinated before entering a Florida school or day care, but it would be easier for parents and guardians to opt out of those vaccinations under a bill passed by the Senate Health Policy Committee Monday afternoon.
-
As part of its 'Year of the Lies' series, PolitiFact FL reports on a pediatrician who quit her long practice of seeing patients in person, after the Trump administration’s unproved claims on everything from Tylenol to vaccines had added chaos and safety concerns to her days.
-
This finding, in conjunction with the University of Texas MD Anderson Cancer Center, comes as the DeSantis administration attempts to ban vaccine mandates, including for public school students.
-
Measles is the most deadly of all childhood rash illnesses, according to the Florida Department of Health. Two children and an adult died this year in a measles outbreak in Texas.
-
In a Sept. 7 interview on CNN’s "State of the Union," Ladapo told host Jake Tapper that his department didn’t study how ending the vaccine requirements could affect children’s health or future outbreaks.
-
The governor announced the state would repeal all vaccine mandates last week.
-
“The ripple effect of removing vaccine entry requirements would affect all of us, not just those with children in school,” said FCAAP President Rana Alissa.
-
Many parents, doctors and other public health workers worry that diseases controlled by vaccines for decades could resurface.
-
The congresswoman's remarks came same day the state's top medical official announced plans to eliminate vaccine mandates.
-
Herd immunity of close to 95% is needed to prevent outbreaks of infectious diseases. And yet, the state continues to see a decline in the vaccination rate among children since the pandemic.
-
The experimental mRNA vaccine developed at the University of Florida has shown the ability to supercharge the immune system and could pave the way for a universal cancer vaccine.
-
While the latest state report shows the first significant decrease in flu cases since the peak, the numbers are still higher compared to the same period in recent years.
Play Live Radio
Next Up:
0:00
0:00
Available On Air Stations